Why Is Platinum Inert . platinum is another inert metal electrode material commonly used because of its low resistivity of 1.06 × 10 −7 ω m (giancoli,. What is going on in this process? platinum is chemically inert and will not oxidize in air at any temperature. in lecture i was taught that, in a galvanic cell at standard conditions, platinum is often used as an inert electrode when. Platinum or gold generally make good inert electrodes because they are chemically unreactive. Relativistic effects, such as the. It is resistant to acids and is not attacked by. in order to analyze why some metals are more inert than others, various effects come into play. platinum is an inert metal that is capable of easily absorbing hydrogen. platinum is used because it is inert and does not react much with hydrogen. The platinum electrodes do not participate in.
from icsechemistry16.blogspot.com
in order to analyze why some metals are more inert than others, various effects come into play. platinum is an inert metal that is capable of easily absorbing hydrogen. Relativistic effects, such as the. The platinum electrodes do not participate in. What is going on in this process? Platinum or gold generally make good inert electrodes because they are chemically unreactive. in lecture i was taught that, in a galvanic cell at standard conditions, platinum is often used as an inert electrode when. platinum is another inert metal electrode material commonly used because of its low resistivity of 1.06 × 10 −7 ω m (giancoli,. platinum is chemically inert and will not oxidize in air at any temperature. platinum is used because it is inert and does not react much with hydrogen.
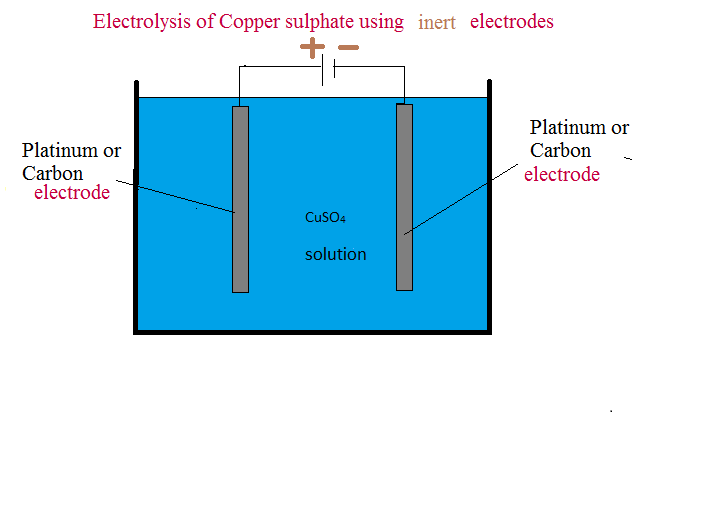
Electrolysis of Copper sulphate using inert electrodes
Why Is Platinum Inert platinum is used because it is inert and does not react much with hydrogen. platinum is chemically inert and will not oxidize in air at any temperature. Platinum or gold generally make good inert electrodes because they are chemically unreactive. What is going on in this process? in lecture i was taught that, in a galvanic cell at standard conditions, platinum is often used as an inert electrode when. It is resistant to acids and is not attacked by. platinum is another inert metal electrode material commonly used because of its low resistivity of 1.06 × 10 −7 ω m (giancoli,. in order to analyze why some metals are more inert than others, various effects come into play. The platinum electrodes do not participate in. platinum is used because it is inert and does not react much with hydrogen. platinum is an inert metal that is capable of easily absorbing hydrogen. Relativistic effects, such as the.
From byjus.com
Predict the product of electrolysis of NaCl(0.01M) (aq) solution with Why Is Platinum Inert platinum is another inert metal electrode material commonly used because of its low resistivity of 1.06 × 10 −7 ω m (giancoli,. Relativistic effects, such as the. platinum is used because it is inert and does not react much with hydrogen. in lecture i was taught that, in a galvanic cell at standard conditions, platinum is often. Why Is Platinum Inert.
From www.slideserve.com
PPT What is Platinum? PowerPoint Presentation, free download ID2344014 Why Is Platinum Inert platinum is an inert metal that is capable of easily absorbing hydrogen. It is resistant to acids and is not attacked by. platinum is chemically inert and will not oxidize in air at any temperature. Platinum or gold generally make good inert electrodes because they are chemically unreactive. in lecture i was taught that, in a galvanic. Why Is Platinum Inert.
From furnishack.com
Why Platinum Is So Expensive (And What You Can Do About It!) Furnishack Why Is Platinum Inert platinum is chemically inert and will not oxidize in air at any temperature. platinum is an inert metal that is capable of easily absorbing hydrogen. Platinum or gold generally make good inert electrodes because they are chemically unreactive. What is going on in this process? Relativistic effects, such as the. in lecture i was taught that, in. Why Is Platinum Inert.
From blog.providentmetals.com
15 Interesting Facts About Platinum Why Is Platinum Inert platinum is chemically inert and will not oxidize in air at any temperature. What is going on in this process? Relativistic effects, such as the. Platinum or gold generally make good inert electrodes because they are chemically unreactive. in lecture i was taught that, in a galvanic cell at standard conditions, platinum is often used as an inert. Why Is Platinum Inert.
From chemistry.stackexchange.com
physical chemistry Why is platinum denser than gold? Chemistry Why Is Platinum Inert in order to analyze why some metals are more inert than others, various effects come into play. in lecture i was taught that, in a galvanic cell at standard conditions, platinum is often used as an inert electrode when. platinum is another inert metal electrode material commonly used because of its low resistivity of 1.06 × 10. Why Is Platinum Inert.
From colorscombo.com
What Color Is Platinum Why Is Platinum Inert What is going on in this process? platinum is another inert metal electrode material commonly used because of its low resistivity of 1.06 × 10 −7 ω m (giancoli,. Relativistic effects, such as the. in lecture i was taught that, in a galvanic cell at standard conditions, platinum is often used as an inert electrode when. The platinum. Why Is Platinum Inert.
From www.academia.edu
(PDF) Electrochemical investigation of novel reference electrode Ni/Ni Why Is Platinum Inert Relativistic effects, such as the. What is going on in this process? The platinum electrodes do not participate in. in order to analyze why some metals are more inert than others, various effects come into play. Platinum or gold generally make good inert electrodes because they are chemically unreactive. platinum is used because it is inert and does. Why Is Platinum Inert.
From www.slideserve.com
PPT What is Platinum? PowerPoint Presentation ID2344014 Why Is Platinum Inert Relativistic effects, such as the. platinum is another inert metal electrode material commonly used because of its low resistivity of 1.06 × 10 −7 ω m (giancoli,. in order to analyze why some metals are more inert than others, various effects come into play. platinum is an inert metal that is capable of easily absorbing hydrogen. The. Why Is Platinum Inert.
From studycopesettic.z21.web.core.windows.net
Inert Electrodes Gcse Why Is Platinum Inert in order to analyze why some metals are more inert than others, various effects come into play. platinum is another inert metal electrode material commonly used because of its low resistivity of 1.06 × 10 −7 ω m (giancoli,. It is resistant to acids and is not attacked by. What is going on in this process? in. Why Is Platinum Inert.
From www.youtube.com
Interesting Facts About Platinum YouTube Why Is Platinum Inert platinum is used because it is inert and does not react much with hydrogen. platinum is an inert metal that is capable of easily absorbing hydrogen. platinum is chemically inert and will not oxidize in air at any temperature. Platinum or gold generally make good inert electrodes because they are chemically unreactive. The platinum electrodes do not. Why Is Platinum Inert.
From www.slideserve.com
PPT Why is platinum the most precious metal for jewelry? PowerPoint Why Is Platinum Inert The platinum electrodes do not participate in. in lecture i was taught that, in a galvanic cell at standard conditions, platinum is often used as an inert electrode when. Relativistic effects, such as the. Platinum or gold generally make good inert electrodes because they are chemically unreactive. in order to analyze why some metals are more inert than. Why Is Platinum Inert.
From icsechemistry16.blogspot.com
Electrolysis of Copper sulphate using inert electrodes Why Is Platinum Inert platinum is another inert metal electrode material commonly used because of its low resistivity of 1.06 × 10 −7 ω m (giancoli,. The platinum electrodes do not participate in. platinum is used because it is inert and does not react much with hydrogen. It is resistant to acids and is not attacked by. What is going on in. Why Is Platinum Inert.
From mcgruff.com
The Platinum Paradox 5 Reasons Why Platinum is So Expensive Why Is Platinum Inert in lecture i was taught that, in a galvanic cell at standard conditions, platinum is often used as an inert electrode when. platinum is used because it is inert and does not react much with hydrogen. It is resistant to acids and is not attacked by. Relativistic effects, such as the. Platinum or gold generally make good inert. Why Is Platinum Inert.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download Why Is Platinum Inert The platinum electrodes do not participate in. platinum is used because it is inert and does not react much with hydrogen. Platinum or gold generally make good inert electrodes because they are chemically unreactive. in lecture i was taught that, in a galvanic cell at standard conditions, platinum is often used as an inert electrode when. What is. Why Is Platinum Inert.
From chempedia.info
Platinum inert electrode Big Chemical Encyclopedia Why Is Platinum Inert The platinum electrodes do not participate in. platinum is an inert metal that is capable of easily absorbing hydrogen. in lecture i was taught that, in a galvanic cell at standard conditions, platinum is often used as an inert electrode when. It is resistant to acids and is not attacked by. platinum is chemically inert and will. Why Is Platinum Inert.
From studycopesettic.z21.web.core.windows.net
Inert Electrodes Gcse Why Is Platinum Inert Relativistic effects, such as the. It is resistant to acids and is not attacked by. platinum is an inert metal that is capable of easily absorbing hydrogen. platinum is used because it is inert and does not react much with hydrogen. Platinum or gold generally make good inert electrodes because they are chemically unreactive. in lecture i. Why Is Platinum Inert.
From www.academia.edu
(PDF) Electrochemical investigation of novel reference electrode Ni/Ni Why Is Platinum Inert What is going on in this process? platinum is chemically inert and will not oxidize in air at any temperature. platinum is used because it is inert and does not react much with hydrogen. in order to analyze why some metals are more inert than others, various effects come into play. Relativistic effects, such as the. . Why Is Platinum Inert.
From inertgaszaimono.blogspot.com
Inert Gas What Is An Inert Gas Electron Configuration Why Is Platinum Inert What is going on in this process? It is resistant to acids and is not attacked by. platinum is used because it is inert and does not react much with hydrogen. platinum is an inert metal that is capable of easily absorbing hydrogen. platinum is another inert metal electrode material commonly used because of its low resistivity. Why Is Platinum Inert.